Combination Therapy of Dinutuximab and Tipifarnib for High-Risk Neuroblastoma
ID# 2021-5356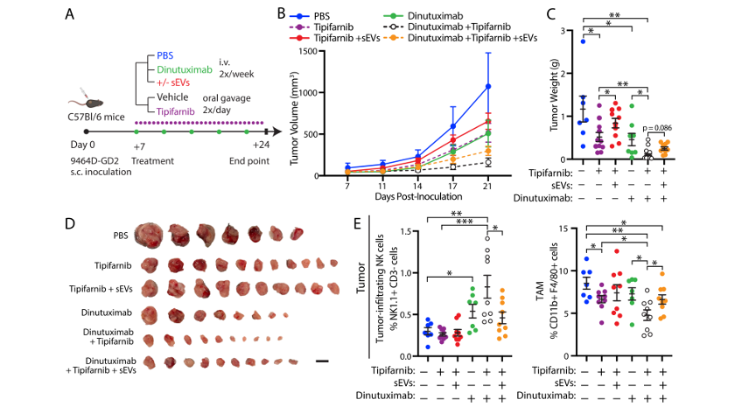
Technology Summary
The inventors showed that neuroblastoma-derived small extracellular vesicles (sEVs) weakened dinutuximab’s efficacy in vivo, altering tumor immune cell infiltration and creating an immunosuppressive microenvironment with more tumor-associated macrophages and fewer tumor-infiltrating NK cells. In addition, these sEVs hindered splenic NK cell maturation in vivo and dinutuximab-induced NK cell-mediated cytotoxicity in vitro. Notably, tipifarnib improved dinutuximab-mediated tumor growth inhibition and countered neuroblastoma-derived sEVs’ immunosuppressive effects in vivo.
Application & Market Utility
The disclosed invention holds significant and transformative practical applications in the realm of treating neuroblastoma patients. In the context of 2023, the estimated market value for neuroblastoma stands at $360 million. Moreover, there exists a promising anticipation for further market expansion, as optimistic projections indicate a robust growth trajectory with the potential for the market to attain a substantial value of $470 million by the year 2032. This innovative breakthrough is poised to make a profound and meaningful impact within this evolving landscape.
Next Steps
The research team is strategically planning to initiate a comprehensive clinical trial to evaluate the promising combination therapy in neuroblastoma patients, with licensing and marketing efforts alongside the study.

