Low-Temperature Liquid-Phase Synthesis of Lithium Thiophosphate-Borohydride Solid Electrolyte with High Room-Temperature Ionic Conductivity
ID# 2023-5587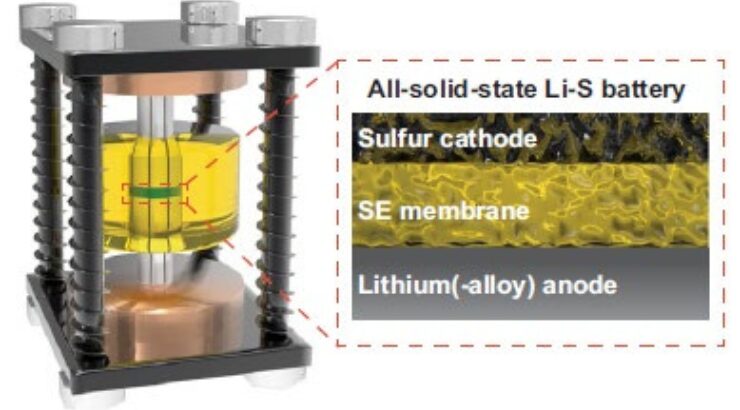
Technology Summary
Lithium-sulfur all-solid-state batteries (Li-S ASSB) using inorganic solidstate electrolytes are considered promising electrochemical energy storage technologies attracting attention as a safe, high-specific-energy, durable, and low-cost power source for potential use in electric vehicles and drones. The ability of inorganic solid electrolytes (SE) to prevent polysulfide dissolution endows Li-S ASSB with potential for achieving higher specific energy and a longer life-span than conventional Li-S batteries using non-aqueous liquid electrolyte solutions. However, developing positive electrodes with high sulfur content, adequate sulfur utilization, and high mass loading is challenging.
Researchers at Penn State have demonstrated a strategy of using low-density, highly ionically conductive SE with small particle size to enable efficient ionic transport in high-sulfur-content cathodes and thus attain Li-S ASSBs with high specific capacity. Researchers further demonstrated that the use of low-density solid electrolyte increases the electrolyte volume ratio in the cathode, reduces inactive bulky sulfur, and improves the content uniformity of the sulfur-based positive electrode. As proof of concept, the argyrodite glass ceramic SE, Li3PS4-2LiBH4 (LPB), was synthesized via a liquid-phase method with a low measured density, high ionic conductivity, and small primary particle size.
Application & Market Utility
The demonstrated strategy enables high-performance Li-S ASSBs with a maximum discharge capacity while also achieving stable cycling with a high initial discharge capacity and a low fading rate. • The global All-Solid-State Battery market was valued at $975.93 million in 2022 and is expected to reach $15419.23 million by 2028 at a CAGR of 58.41%.
Next Steps
The researchers are seeking licensing and research collaboration opportunitites.

