Ozone Oxidative Precipitation of Elements from Aqueous Streams
ID# 2022-5479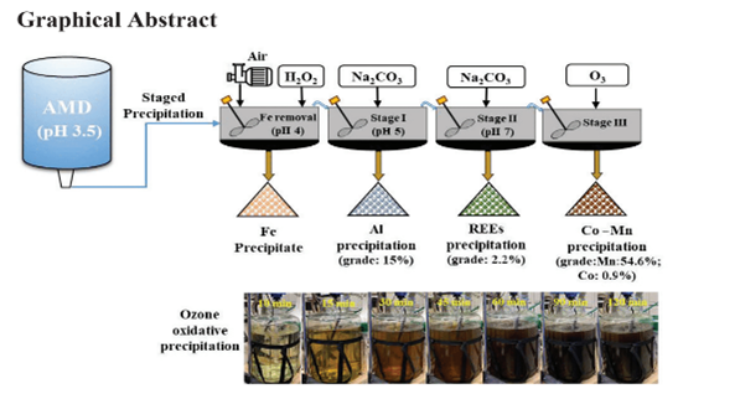
Technology Summary
Researchers at Penn State have developed a chemical-free oxidative precipitation technique for recovery of Co, Mn, Ni, Cu, Ag, Pd, Bi, and Ta from aqueous streams. Available aqueous streams for use in the process include alkaline mine seepage water, pregnant leaching solutions acquired through electronic waste, recycled batteries and/or sludge, acid mine drainage (AMD), and industrial wastewaters. Various ligands (OH–, CO32-, NH4+, SO42-, PO43-) and oxidants (Na2S2O8, KMnO4, O3) were evaluated. Testing indicated that the (NH4+)OH ligands provided the highest recovery of Co and Mn (>98%) and grade for these elements (0.9% Co, 28% Mn). The oxidizer O3 resulted in the highest recovery (>98%) and grade (0.9% Co, 54.6% Mn). Based on these encouraging results, a three-stage precipitation process was designed to selectively recover Co and Mn while neutralizing the AMD to eliminate water pollution. Overall recoveries were > 95% Co-Mn, 90%-95% of the rare earth metals, and 85% of Al.
Application & Market Utility
- The developed ozone oxidative precipitation process for Co and Mn from aqueous streams is chemical-free and capable of being performed over a wide pH range.
- Selective Co and Mn recovery without needing to adjust the pH of aqueous streams minimizes critical metal recovery costs, mitigates adverse environmental effects, and can be used on various waste feedstocks.
- The global Co market was valued at $8.7 billion in 2021 and is expected to reach $19.47 billion by 2030 at a CAGR of 9.3%.
- The global Mn market was valued at $22.1 billion in 2021 and is expected to reach $32.7 billion by 2030 at a CAGR of 5.02%.
Next Steps
Researchers are seeking licensing and research collaboration opportunities.

