Molecular Biomarkers of High-Grade HPV Epithelial Cell Lesions
ID# 2020-5140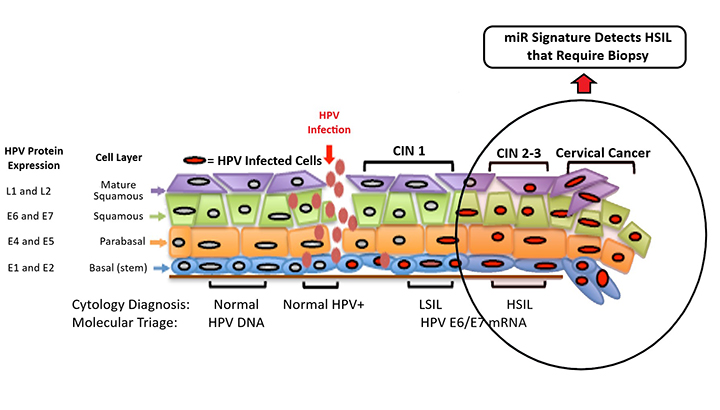
Technology Summary
MicroRNAs (miRs) are particularly promising markers of disease due to their long-term stability and bioavailability in serum, urine, and saliva. This technology utilizes a set of miRs as diagnostic biomarkers to identify high-grade squamous intraepithelial lesions (HSIL) in Pap smears of exfoliated cervical cells, with higher sensitivity than conventional Pap screening protocols. The researchers are actively developing this technology to show that this diagnostic miR signature is also detectable in urine samples.
Application & Market Utility
Cervical cancer is the fourth leading cause of cancer death worldwide, with over 528,000 cases diagnosed annually. Current cervical cancer screening programs rely on the Pap smear, which requires that a medical professional directly sample the uterine cervix with a cytology-brush via an invasive speculum exam for diagnosis by a cytopathologist. While organized Pap screening protocols have significantly decreased the morbidity and mortality associated with cervical cancer, the incidence of cervical cancer in low-resource settings remains unchanged, as lack of access to medical expertise, high costs, and time constraints prevent women from being screened. A urine-based screening test for HSIL would be a non-invasive option that could broaden access to life-saving cervical cancer screening in both low-resource medical settings and also kits for at-home use.
Next Steps
Seeking research collaboration and licensing partners.

