One-Step Catalytic Transformation of Carbohydrates to Liquid Fuels
ID# 2008-3460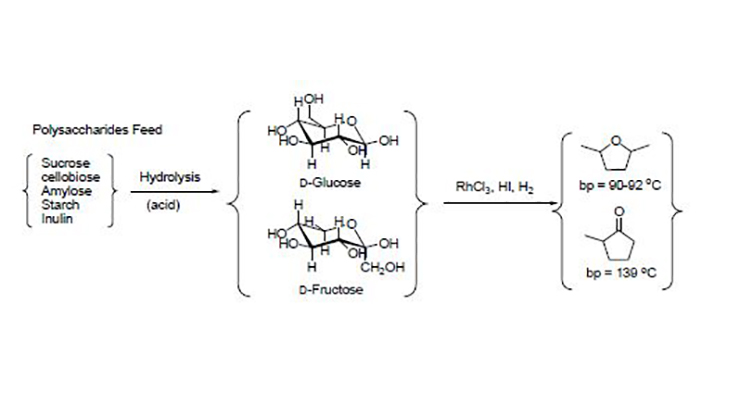
Technology Summary
Ethanol production suffers from low energy density, high volatility and contamination by the absorption of water from the surrounding environment. 2,5-dimethylfuran (DMF), an alternative fuel, has advantages over ethanol, including a higher energy density, a higher boiling point, and lower solubility in water. Previous production methods of DMF involved expensive, energy-consuming separation steps.
The subject invention represents a one step transformation from carbohydrate containing biomass source material to dimethyltetrahydrofuran (DMTHF), which has a higher energy content and provides additional storage stability than DMF. The feedstock can be simple sugars or more complex carbohydrates. The invention includes catalytic compositions specific for this process.
Application & Market Utility
The researchers demonstrated that this invention’s process has high yield and high selectivity. The processing temperature ranged around 30oC to 120oC with or without a solvent. Pressures did not exceed 600 psi. The transformation from fructose to DMTHF occurred within two to four (2-4) hours. The separation of the liquid fuels from aqueous solution is fairly straightforward, thereby allowing the energy efficiency to be really high. Additionally, the process can also produce 2-methylcyclopentanone (MCPO), which is another higher energy density liquid fuel candidate.
Next Steps
Seeking licensing opportunities of issued US Patent Nos. 8,440,870 and 8,674,150.

