System for Rapid Antimicrobial Susceptibility Testing and Pathogen ID
ID# 2018-4789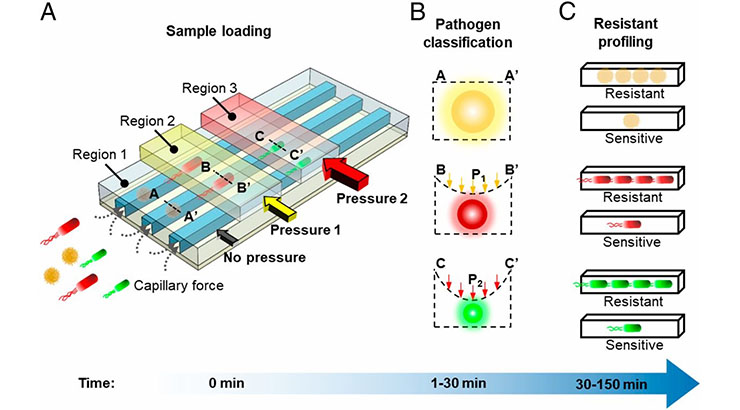
Technology Summary
This technology relates to an adaptable microfluidic system for rapid pathogen classification and antimicrobial susceptibility testing at the single-cell level. By incorporating tunable microfluidic valves and real-time optical detection, bacteria can be trapped and classified based on their physical shape and size. By monitoring their growth in the presence of antibiotics at the single cell level, antimicrobial susceptibility of the bacteria can be determined in as little as 30 minutes. The figure above shows: (A) a schematic of the microfluidic device, showing bacterial pathogens loaded into the channels by capillary action; (B) cross-section profiles of a channel under different pneumatic pressures trapping bacteria in different regions of the channel; and (C) antimicrobial susceptibility determined by monitoring phenotypic growth of the bacteria in the presence of antibiotics.
Application & Market Utility
The key advantage of this technology is that antimicrobial susceptibility can be determined in as little as 30 minutes, compared to current procedures that take days, which will profoundly impact the clinical management of bacterial infections. Bacterial pathogens can be detected in urine, blood cultures, and whole blood, and polymicrobial samples can be analyzed. In a pilot study of 25 clinical urine samples, the system demonstrated 100% sensitivity and 83.33% specificity for pathogen classification with 100% concordance for antimicrobial susceptibility testing.
Next Steps
Seeking research collaboration and licensing opportunities.

