System to Measure Stability of Concentrated Therapeutic Proteins
ID# 2016-4476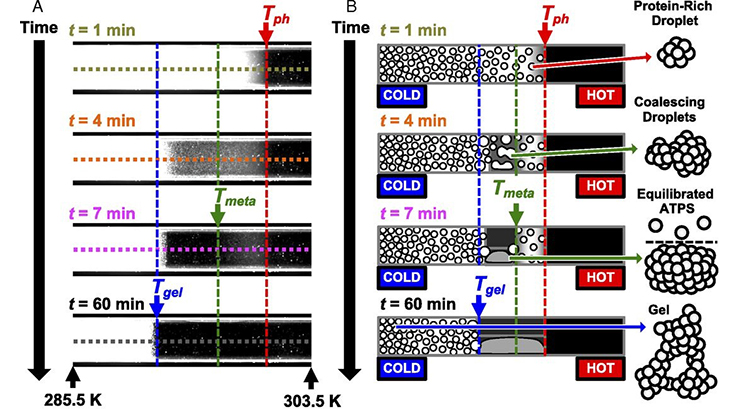
Technology Summary
Liquid formulations of therapeutic proteins require storage at high concentration and must remain stable for 2 years for FDA approval. Drug formulation is hindered by slow and inaccurate tests for predicting a drug’s shelf life. This technology offers high-throughput and accurate predictions of colloidal stability in therapeutic protein formulations that will reduce formulation screening time from 1 month to 1 day. Current prototypes employ two thermoelectric cooling plates – across which the samples loaded in glass capillaries are placed – to form a temperature gradient across the samples, and dark-field microscopy is used to image the samples. Image analysis is used to extract key thermodynamic and kinetic parameters from the images for each experiment. The figure above shows a mAb solution at various time points (left) and schematically depicts the stages of phase separation (right).
Application & Market Utility
The main advantage of this technology is its ability to measure protein phase behavior over a range of temperatures and solution conditions simultaneously, saving considerable time for drug developers by allowing rapid screening of solution conditions and drug candidates. Other advantages of this technology include the potential for multiplexed detection capabilities (i.e., testing multiple samples simultaneously), a small sample volume requirement, and the ability to test the exact protein formulation of interest – even at very high protein concentrations.
Next Steps
Seeking research collaboration and licensing opportunities.

