Topical Naltrexone as a Treatment for Dry Eye
ID# 2008-3489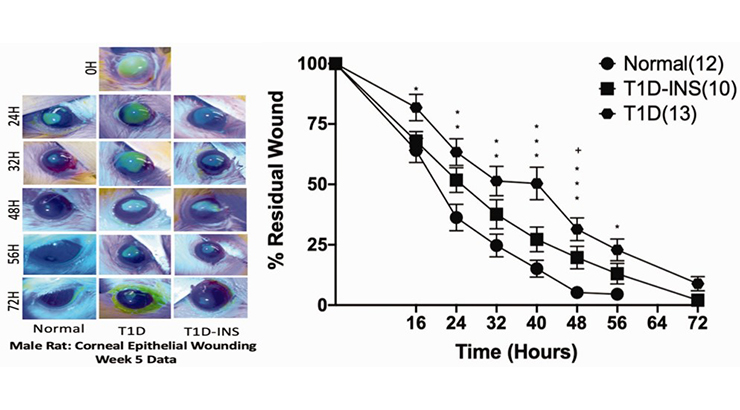
Technology Summary
Diabetes often presents with systemic ocular surface complications including dry eye, keratopathy, and eye sensitivity. Studies have indicated 54% prevalence of asymptomatic and symptomatic Dry Eye Syndrome in diabetes. Penn State inventors have demonstrated that dysregulation of opioid growth factor (OGF)–OGF receptor (OGFr) regulation in the diabetic cornea is associated with the onset and magnitude of ocular surface complications. Dysregulation of the OGF–OGFr axis is present in both uncontrolled and insulin-controlled diabetic animal models. Penn State inventors have shown that the OGF–OGFr axis can be modulated by Naltrexone (NTX), a long-acting opioid receptor antagonist that is currently FDA approved for the management of drug, alcohol, and nicotine dependence. Topical application of NTX enhances corneal repair and increases tear production in type 1 diabetic animal models. Topical application of NTX also resulted in the return of corneal hypersensitivity and increased tear production in type 2 diabetes animal models. The inventors have further established a novel, non-toxic eye drop formulation of NTX.
Application & Market Utility
Dry eye is a disorder of increasing prevalence that affects tear production. Chronic dryness leads to pain and irritation and often impedes normal daily activities. Sustained dry eye can lead to permanent damage and result in vision loss, increased risk of infection, and scarring of the cornea. Currently, the most frequently prescribed therapy by vision specialists is tear supplementation (e.g., lubricants). Lubricants do not treat the cause of dry eye, can be expensive, and need to be taken for life.
Next Steps
Phase I clinical validation of naltrexone for the treatment of dry eye is complete. A protocol for Phase II is available. Penn State seeks an industry partner and/or licensee to complete US clinical validation.

