Topical Naltrexone Treatment for Non-Healing Diabetic Foot Ulcers
ID# 2009-3563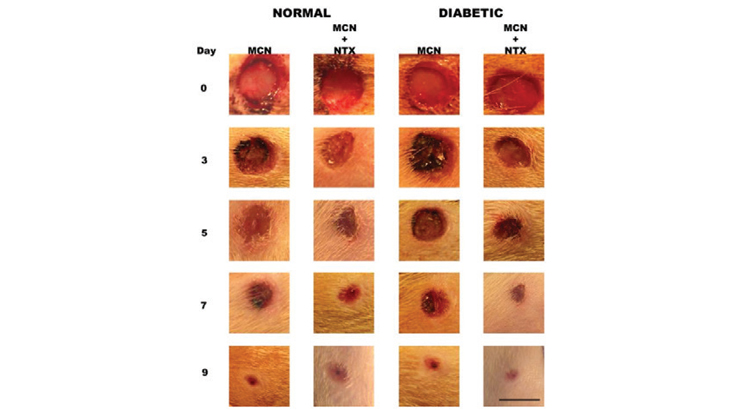
Technology Summary
For diabetic patients, non-healing foot ulcers are common and can result in complications (worsening of the ulceration, systemic infection, severe pain, amputation). This technology is a topical administration of naltrexone (NTX) dissolved in a cream, to continuously block the Opioid Growth Factor Receptor sites on wounded tissue. Animal studies show a 25-30% reduction in time for wound healing with NTX. This approach to wound healing specifically enhances new cell replication by increasing DNA synthesis resulting in increased angiogenesis and granulation in epithelial tissue, as well as reducing complications such as infection related to delayed wound closure. NTX accelerates wound closure both in diabetic and non-diabetic patients, making it an optimal treatment to increase the effectiveness of wound repair throughout the population.
Application & Market Utility
There are 26 million diabetics and 86 million pre-diabetics in the U.S. There is 285 million diabetics worldwide. 4.5 million diabetics develop DFUs annually with a U.S. DFU healthcare cost exceeding $20B annually. The current treatment is costly, has side effects, and carries risk of death following long use. Over-the-counter bandages and creams are ineffective. Topical application of NTX provides fast, inexpensive and safe treatment to accelerate closure of cutaneous wounds. End users: diabetics, healthy individuals, and veterinary practitioners.
Next Steps
Seeking research collaboration and licensing opportunities.

