Success Story
“Without the opportunities of the Startup Leadership Network and the QED Program, we would not be able to move our ideas into the marketplace."
Dr. Patricia McLaughlin
Penn State Professor and ElkosRx, LLC Cofounder
Penn State Researchers Develop Topical Treatment for Diabetic Wound Healing
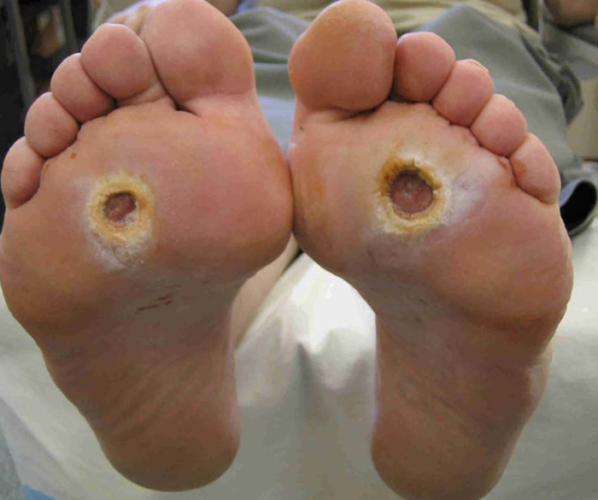
Every year 73,000 diabetic patients in the United States receive the devastating news that they must undergo a lower limb amputation. Surprisingly, 85% of these cases start out with a seemingly non-threatening diabetic foot ulcer (DFU), an open sore that’s usually located on the bottom of the foot. One in four diabetics suffer from DFUs which quickly can become a non-healing chronic wound that jeopardizes the entire limb. Once progressed to a later stage DFU, treatment options become quite difficult to administer and very expensive.
Development of new therapies for early stage DFUs has stalled over the past 20 years. The current standard-of-care treatments consist of basic wound care and offloading of the affected area. Efficacy is sub-optimal and treatment fails to address the underlying fundamental pathophysiological processes behind DFUs.
Drs. Patricia McLaughlin, Joseph Sassani, and Ian Zagon at Penn State College of Medicine have been studying the opioid growth regulatory system for decades, a prime factor for determining cell biogenesis and division, physiological homeostasis, development, wound healing and disease. They have discovered that both Type 1 and Type 2 diabetes cause a dysfunction of the opioid growth regulatory system resulting in upregulation of the negative growth factor, which leads to suppressed wound healing. Their research also found that the opioid antagonist NTX will act as a blockade to the opioid growth factor receptor pathway, restoring cells to a normal wound healing state.
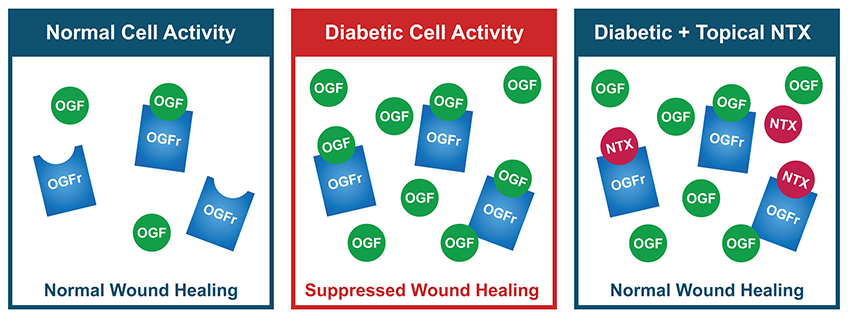
Preclinical studies have demonstrated and confirmed that applying topical NTX to diabetic wounds enhances healing of full thickness cutaneous wounds by targeting the dysfunctional opioid growth regulatory pathway. Animal studies have further demonstrated that NTX increases epithelial cell division, stimulates new blood vessel formation, and improves collagen production, which accelerates the rate of wound closure and strengthens damaged skin.
With funding from the Penn State Research Foundation and College of Medicine and assistance from the Center for Medical Innovation, the research team has been able to partner with a pharmaceutical company to develop a novel topical therapy and further develop their technology with a goal of making it commercially competitive. Compared to current treatments, they believe their therapeutic solution is safer, less expensive, more efficacious and can be administered by the patient or caregiver in the home.
“It’s quite exciting to know we can go from bench to bedside and really make a difference in the quality of life for diabetics,” says Dr. Mclaughlin.
Scientific merits for their approach have been recognized through significant technical and/or financial support from the American Diabetes Association, NIH, Penn State’s Fund for Innovation, and the University City Science Center QED Proof-of-Concept Program. In particular, the Fund for Innovation will support the manufacturing of Good Manufacturing Practice (GMP) material necessary for clinical research.
Recently, Drs. McLaughlin, Sassani and Zagon partnered with Dr. Mark Lane, an accomplished business partner and drug development lead they met through Invent Penn State’s Startup Leadership Network. Together they formed ElkosRx, LLC, a startup company aimed at raising seed capital to take topical NTX into clinical trial – which will move them one step closer to getting forthcoming patients back on their feet.
“Under the leadership of Mark, we have several strategies in place to complete the required preclinical studies, submit an IND, and move into commercialization of the topical therapy for DFUs,” says Dr. McLaughlin. “Without the opportunities of the Startup Leadership Network and the QED Program, we would not be able to move our ideas into the marketplace. As scientists and clinicians, we’re dependent on the business expertise of others and the Startup Leadership Network provides such collaborators. We are very pleased to have received the support of the QED business and legal experts, as well as partnering with Mark Lane on business opportunities to raise capital and/or receive funding for clinical trials.“