Inhalable Antimicrobial Peptide Particles
ID# 2017-4634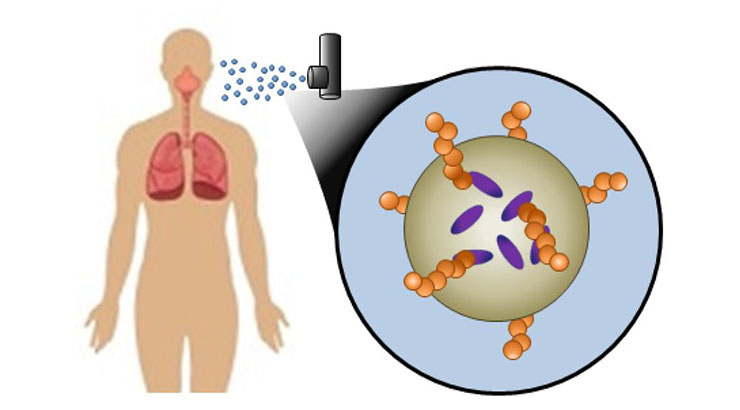
Technology Summary
The present invention is directed to a new class of inhalable drug delivery particle gels that are prepared through the assembly of antimycobacterial peptides (AMPs) and FDA approved carbohydrates. Combinatorial therapies can be stably loaded and controllably released without the need for harsh chemicals or cross-linking reactions. After a patient inhales the particles, the technology allows for the recruitment of bacterial pathogens and targeted delivery of loaded AMPs and antibiotics to elicit sustained antimicrobial responses towards drug resistant bacteria. The permeabilization of bacterial membranes by AMPs can enhance the uptake of delivered drugs, thereby increasing their potency. Most AMPs can also be prepared quickly, and at low cost due to their short sequences.
Application & Market Utility
Respiratory diseases such as tuberculosis (TB), pneumonia, and infections caused by cystic ?brosis, may represent target indications of the present invention. Due to the unique mechanism of action, the inventors believe this technology may be uniquely suited to addressing TB and drug-resistant TB, a leading cause of mortality worldwide. Per the CDC, in 2017 an estimated 10 million people contracted TB and over 1.3 million people suffered TB-related deaths worldwide. The WHO cites that over 550,000 people in 2017 were diagnosed with multidrug or rifampicin resistant TB. Rise of drug resistant TB infections has a created an urgent market need for novel therapies that address current treatment shortfalls, including burdensome treatment regimens and drug intolerance or toxicity.
Next Steps
Samples of the technology can be provided. Seeking licensing opportunities and research collaboration. Research is ongoing.

