Sustained-Release Protein Therapy for Tissue Regeneration
ID# 2015-4383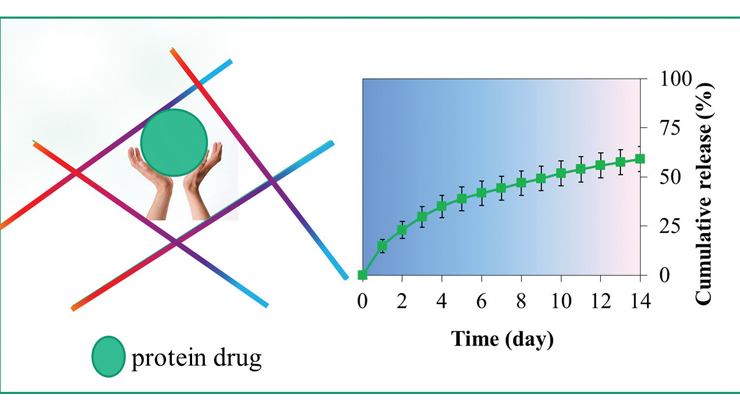
Technology Summary
The invention describes a combination of materials and a method of preparation of a biocompatible porous composite functionalized with aptamers that can bind to an active agent (such as chemokines, interleukins, growth factors, or any other biologically active molecule). Existing biomaterials for tissue regeneration contain protein drugs. However, current FDA-approved products release protein drugs too rapidly, which dramatically reduces therapeutic effectiveness and causes significant side effects. An ideal product for clinicians to use needs to release protein drugs on-demand, with prolonged therapy and minimal side effects. By contrast, this technology releases protein drugs in a sustained manner. It can be readily customized to tune the dose of protein drugs by clinicians to meet the needs of treatment.
Application & Market Utility
This technology is a biomaterial that can be developed into different forms such as a solution, a pad, or a cube to match the conditions of wounds and according to the need of clinicians. It is an “off-the-shelf” material that can be shipped and stored at room temperature without the need of a refrigerator.
Next Steps
Seeking research collaboration and licensing opportunities.

